Goat Anti-Equine IgG(H+L)-HRP
Cat. No.:
6040-05
Goat Anti-Equine IgG(H+L)-HRP antibody for use in ELISA, immunocytochemistry, and western blot assays.
$81.00
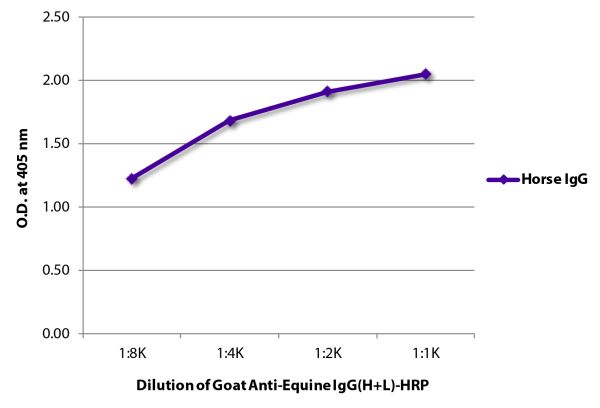

| Isotype | Goat IgG |
|---|---|
| Isotype Control | 0109-05 |
| Specificity | Reacts with the heavy and light chains of equine IgG |
| Source | Pooled antisera from goats hyperimmunized with equine IgG |
| Cross Adsorption | None; may react with immunoglobulins from other species and the light chains of other equine immunoglobulins |
| Purification Method | Affinity chromatography on equine IgG covalently linked to agarose |
| Conjugate | HRP (Horseradish Peroxidase) |
| Buffer Formulation | 50% Glycerol/50% Phosphate buffered saline, pH 7.4 |
| Clonality | Polyclonal |
| Concentration | Lot specific |
| Volume | 1.0 mL |
| Recommended Storage | 2-8°C |
| Applications |
Quality tested applications for relevant formats include - ELISA 1-3 FLISA Other referenced applications for relevant formats include - Flow Cytometry 4,5 Immunocytochemistry 6 Western Blot 7-10 |
| RRID Number | AB_2796117 |
Documentation
Certificate of Analysis Lookup
Enter the Catalog Number and Lot Number for the Certificate of Analysis you wish to view
- 1. Hobo S, Niwa H, Anzai T, Jones JH. Changes in serum antibody levels after vaccination for strangles and after intranasal challenge with Streptococcus equi subsp. equi in horses. J Equine Sci. 2010;21:33-7. (ELISA)
- 2. De Keyser K, Oosterlinck M, Raes E, Ducatelle R, Janssens S, Buys N. Early detection of chronic progressive lymphoedema susceptibility in Belgian draught horse stallions by means of ELISA. Commun Agric Appl Biol Sci. 2012;77:183-7. (ELISA)
- 3. De Keyser K, Berth M, Christensen N, Willaert S, Janssens S, Ducatelle R, et al. Assessment of plasma anti-elastin antibodies for use as a diagnostic aid for chronic progressive lymphoedema in Belgian Draught Horses. Vet Immunol Immunopathol. 2015;163:16-22. (ELISA)
- 4. Colson YL, Li H, Boggs SS, Patrene KD, Johnson PC, Ildstad ST. Durable mixed allogeneic chimerism and tolerance by a nonlethal radiation-based cytoreductive approach. J Immunol. 1996;157:2820-9. (FC)
- 5. Flynn J, Cox CV, Rizzo S, Foukaneli T, Rice K, Murphy M, et al. Direct binding of antithymoctye globulin to haemopoietic progenitor cells in aplastic anaemia. Br J Haematol. 2003;122:289-97. (FC)
- 6. Toscan G, Cadore GC, Pereira RC, Silva GB, Cezar AS, Sangioni LA, et al. Equine neosporosis: occurrence of antibodies against Neospora spp. and association between the serological status of the mares and of their offspring. Pesq Vet Bras. 2010;30:641-5. (ICC)
- 7. van 't Veer C, Mann KG. Regulation of tissue factor initiated thrombin generation by the stoichiometric inhibitors tissue factor pathway inhibitor, antithrombin-III, and heparin cofactor-II. J Biol Chem. 1997;272:4367-77. (WB)
- 8. Warner S, Hartley CA, Stevenson RA, Ficorilli N, Varrasso A, Studdert MJ, et al. Evidence that Equine rhinitis A virus VP1 is a target of neutralizing antibodies and participates directly in receptor binding. J Virol. 2001;75:9274-81. (WB)
- 9. Campbell JE, Brummel-Ziedins KE, Butenas S, Mann KG. Cellular regulation of blood coagulation: a model for venous stasis. Blood. 2010;116:6082-91. (WB)
- 10. Zhang J, Meng W, Wang C, Wu Z, Wu G, Xu Y. Expression, purification and characterization of recombinant plasminogen activator from Gloydius brevicaudus venom in Escherichia coli. Protein Expr Purif. 2013;91:85-90. (WB)
See More