Goat Anti-Canine IgG(H+L)-BIOT
Cat. No.:
6070-08
Goat Anti-Canine IgG(H+L)-Biotin antibody for use in ELISA, immunohistochemistry / immunocytochemistry, and western blot assays.
$88.00
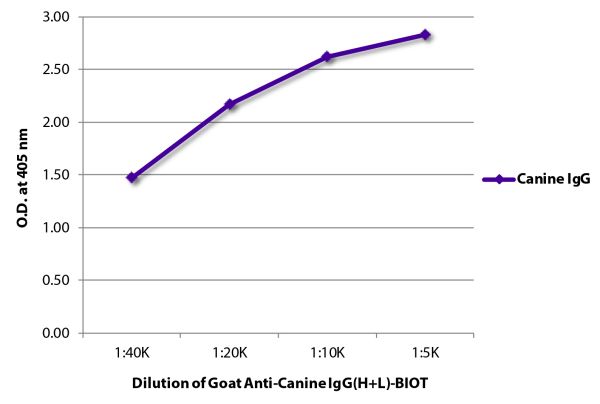

| Isotype | Goat IgG |
|---|---|
| Isotype Control | Goat IgG-BIOT |
| Specificity | Reacts with the heavy and light chains of canine IgG |
| Source | Pooled antisera from goats hyperimmunized with canine IgG |
| Cross Adsorption | None; may react with immunoglobulins from other species and the light chains of other canine immunoglobulins |
| Purification Method | Affinity chromatography on canine IgG covalently linked to agarose |
| Conjugate | BIOT (Biotin) |
| Buffer Formulation | Phosphate buffered saline containing < 0.1% sodium azide |
| Clonality | Polyclonal |
| Concentration | 0.5 mg/mL |
| Volume | 2.0 mL |
| Recommended Storage | 2-8°C |
| Applications |
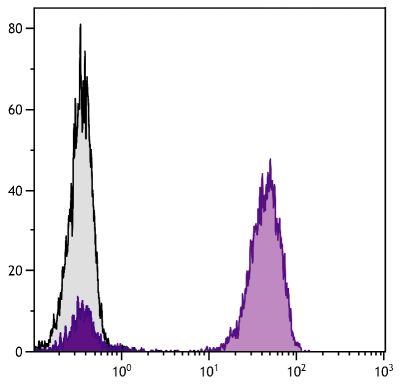
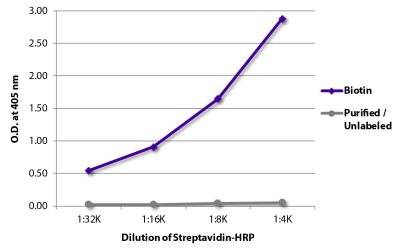
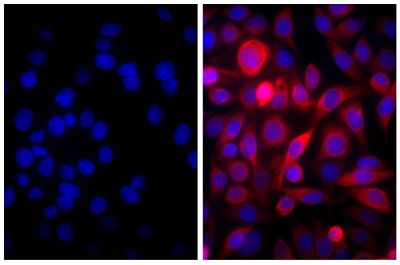
Quality tested applications for relevant formats include - ELISA 1,2 FLISA Other referenced applications for relevant formats include - Immunohistochemistry-Frozen Sections 3 Immunocytochemistry 4 Western Blot 5-8 |
| RRID Number | AB_2796148 |
Documentation
Certificate of Analysis Lookup
Enter the Catalog Number and Lot Number for the Certificate of Analysis you wish to view
- 1. Kakkis E, Lester T, Yang R, Tanaka C, Anand V, Lemontt J, et al. Successful induction of immune tolerance to enzyme replacement therapy in canine mucopolysaccharidosis I. Proc Natl Acad Sci USA. 2004;101:829-34. (ELISA)
- 2. Dickson P, Peinovich M, McEntee M, Lester T, Le S, Krieger A, et al. Immune tolerance improves the efficacy of enzyme replacement therapy in canine mucopolysaccharidosis I. J Clin Invest. 2008;118:2868-76. (ELISA)
- 3. Wiberg ME, Saari SA, Westermarck E, Meri S. Cellular and humoral immune responses in atrophic lymphocytic pancreatitis in German shepherd dogs and rough-coated collies. Vet Immunol Immunopathol. 2000;76:103-15. (IHC-FS)
- 4. Ulchar I, Celeska I, Stefanovska J, Jakimovska A. Hematological and biochemical parameters in symptomatic and asymptomatic leishmania-seropositive dogs. Mac Vet Rev. 2015;38:i-viii. (ICC)
- 5. Mathis A, Åkerstedt J, Tharaldsen J, Ødegaard Ø, Deplazes P. Isolates of Encephalitozoon cuniculi from farmed blue foxes (Alopex lagopus) from Norway differ from isolates from Swiss domestic rabbits (Oryctolagus cuniculus). Parasitol Res. 1996;82:727-30. (WB)
- 6. Rasmussen UB, Benchaibi M, Meyer V, Schlesinger Y, Schughart K. Novel human gene transfer vectors: evaluation of wild-type and recombinant animal adenoviruses in human-derived cells. Hum Gene Ther. 1999;10:2587-99. (WB)
- 7. Müller-Doblies UU, Herzog K, Tanner I, Mathis A, Deplazes P. First isolation and characterisation of Encephalitozoon cuniculi from a free-ranging rat (Rattus norvegicus). Vet Parasitol. 2002;107:279-85. (WB)
- 8. Burk SV. Detection of antibodies against Parascaris equorum excretory-secretory antigens [dissertation]. Lexington (USA): University of Kentucky, 2013 (WB)
See More