Human Type V Collagen-Solution
Cat. No.:
1270-01S
Purified Human Type V Collagen solution for use as a coating material and standard.
$335.00
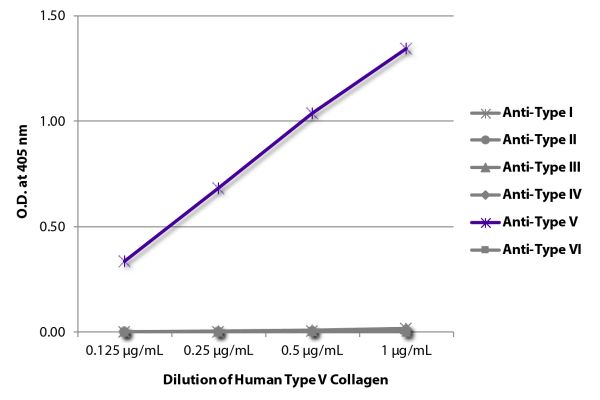

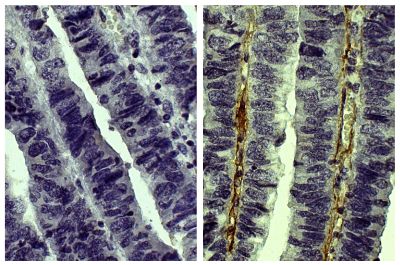
| Description | Collagen is the main structural protein in the extracellular space and is the most abundant protein in the ECM. Collagens are divided into two classes - fibril (types I, II, III, V) and non-fibril (types IV, VI). Type V collagen is a minor connective tissue component of nearly ubiquitous distribution. Type V collagen mutations are associated with Ehlers-Danlos syndrome. Type V collagen is broadly expressed as a two α1(V) chains and one α2(V) chain heterotrimer but also as a α1(V), α2(V), and α3(V) heterotrimer in pancreatic islets, adipose tissue, and skeletal muscle. |
|---|---|
| Source | Placental villi |
| Purity | > 90% by SDS-PAGE |
| Purification Method | Controlled and limited pepsin digestion followed by selective salt precipitation |
| Buffer Formulation | 500 mM acetic acid |
| Concentration | 0.5 mg/mL |
| Volume | 0.5 mL |
| Recommended Storage | 2-8°C |
| Applications |
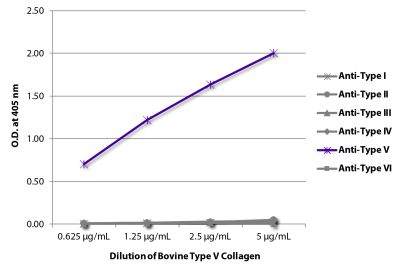
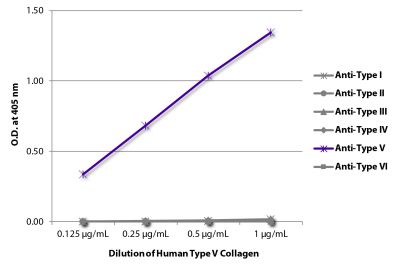
ELISA – Quality tested 1-3 SDS-PAGE – Quality tested Thermal Stability Studies – Reported in literature 4 Coating Material for – Adhesion Studies – Reported in literature 2-3 ECM Interaction Studies – Reported in literature 5,6 |
Documentation
Certificate of Analysis Lookup
Enter the Catalog Number and Lot Number for the Certificate of Analysis you wish to view
- 1. Bumann A, Carvalho RS, Schwarzer CL, Yen EH. Collagen synthesis from human PDL cells following orthodontic tooth movement. Eur J Orthod. 1997;19:29-37. (ELISA, Standard Curve)
- 2. Saito K, Tanaka T, Kanda H, Ebisuno Y, Izawa D, Kawamoto S, et al. Gene expression profiling of mucosal addressin cell adhesion molecule-1+ high endothelial venule cells (HEV) and identification of a leucine-rich HEV glycoprotein as a HEV marker. J Immunol. 2002;168:1050-9. (ELISA, Coat, Adhesion Studies)
- 3. Nagakubo D, Murai T, Tanaka T, Usui T, Matsumoto M, Sekiguchi K, et al. A high endothelial venule secretory protein, mac25/angiomodulin, interacts with multiple high endothelial venule-associated molecules including chemokines. J Immunol. 2003;171:553-61. (ELISA, Coat, Adhesion Studies)
- 4. Makareeva E, Mertz EL, Kuznetsova NV, Sutter MB, DeRidder AM, Cabral WA, et al. Structural heterogeneity of type I collagen triple helix and its role in osteogenesis imperfecta. J Biol Chem. 2008;283:4787-98. (Thermal Stability Studies)
- 5. Bidanset DJ, Guidry C, Rosenberg LC, Choi HU, Timpl R, Hook M. Binding of the proteoglycan decorin to collagen type VI. J Biol Chem. 1992;267:5250-6. (Coat, ECM Interaction Studies)
- 6. Hocking AM, Strugnell RA, Ramamurthy P, McQuillan DJ. Eukaryotic expression of recombinant biglycan. Post-translational processing and the importance of secondary structure for biological activity. J Biol Chem. 1996;271:19571-7. (Coat, ECM Interaction Studies)
See More