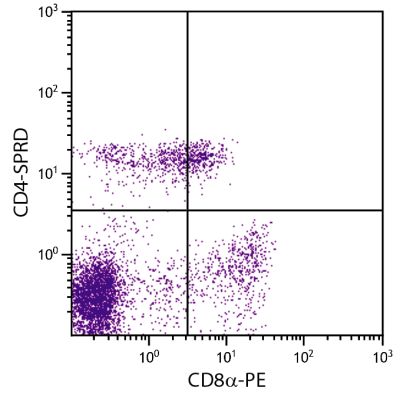
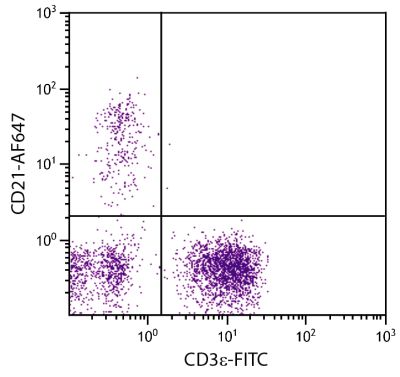
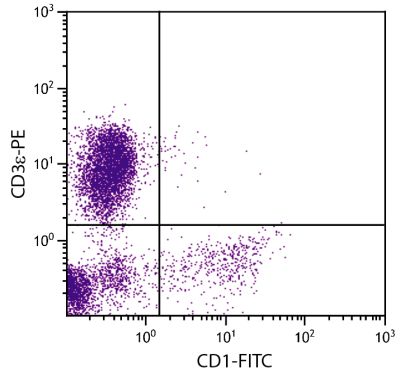
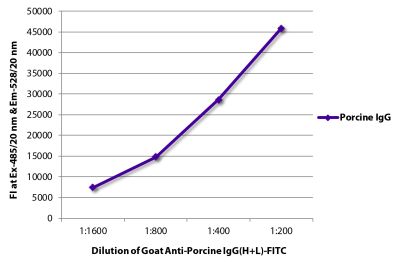
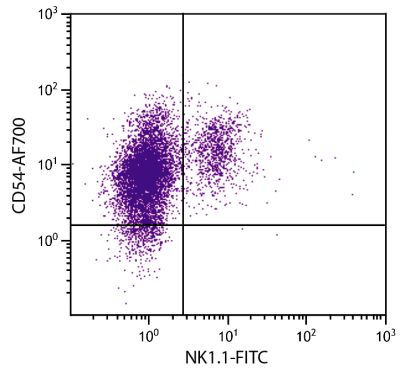
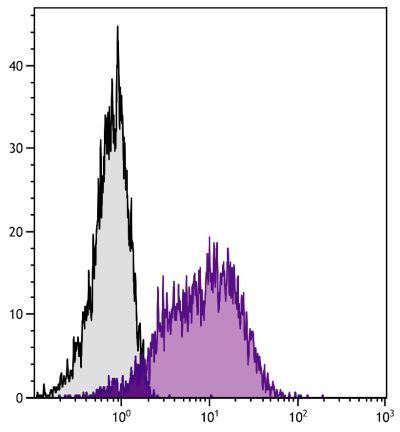
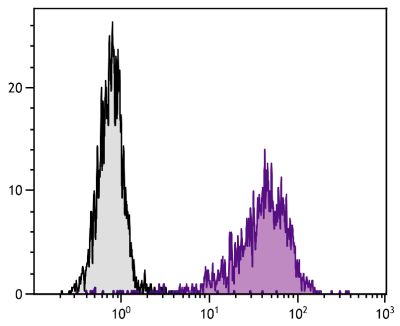
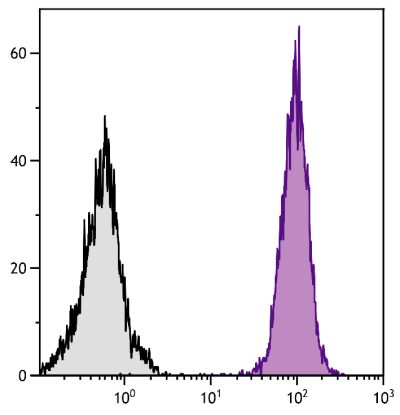
Mouse Anti-Porcine CD8α-PE (76-2-11)
Cat. No.:
4520-09
PE Anti-Porcine CD8α for use in flow cytometry assays.
$262.00
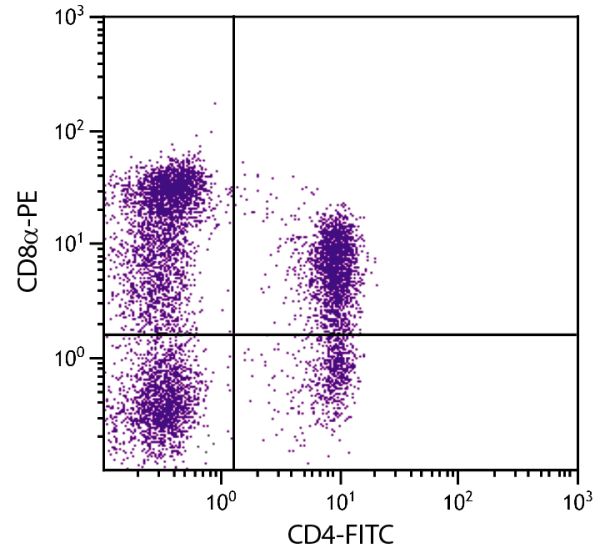

| Clone | 76-2-11 |
|---|---|
| Isotype | Mouse (BALB/c) IgG2aκ |
| Isotype Control | Mouse IgG2a-PE (HOPC-1) |
| Specificity | Porcine CD8α |
| Description | Porcine CD8, a type I transmembrane glycoprotein, is expressed as either a CD8αα homodimer or a CD8αβ heterodimer. It is found on most thymocytes and on the suppressor/cytotoxic subpopulation of peripheral T cells. CD8 functions primarily as a co-receptor with MHC Class I-restricted T cell receptors in antigen recognition. The monoclonal antibody 76-2-11 reacts with the CD8 α-chain. |
| Immunogen | Fresh dd miniature swine thymocytes |
| Conjugate | PE (R-phycoerythrin) |
| Buffer Formulation | Phosphate buffered saline containing < 0.1% sodium azide and a stabilizer |
| Clonality | Monoclonal |
| Concentration | 0.1 mg/mL |
| Volume | 1.0 mL |
| Recommended Storage | 2-8°C; Avoid exposure to light; Do not freeze |
| Applications |
Flow Cytometry – Quality tested 1,11-15,17-28 Immunohistochemistry-Frozen Sections – Reported in literature 2-6,16 Immunohistochemistry-Paraffin Sections – Reported in literature 7 Immunoprecipitation – Reported in literature 1 Blocking – Reported in literature 8,9 Depletion – Reported in literature 10 Complement Mediated Cell Depletion – Reported in literature 1 |
| RRID Number | AB_2796033 |
| Gene ID |
396627 (Porcine) |
| Gene ID Symbol |
CD8A (Porcine) |
Documentation
Certificate of Analysis Lookup
Enter the Catalog Number and Lot Number for the Certificate of Analysis you wish to view
- 1. Pescovitz MD, Lunney JK, Sachs DH. Preparation and characterization of monoclonal antibodies reactive with porcine PBL. J Immunol. 1984;133:368-375. (Immunogen, FC, IP, CMDC)
- 2. Minguez I, Rueda A, Domínguez J, Sánchez-Vizcaíno JM. Double labeling immunohistological study of African swine fever virus-infected spleen and lymph nodes. Vet Pathol. 1988;25:193-8. (IHC-FS)
- 3. Yamada K, Shimizu A, Ierino FL, Utsugi R, Barth RN, Esnaola N, et al. Thymic transplantation in miniature swine. I. Development and function of the "thymokidney". Transplantation. 1999;68:1684-92. (IHC-FS)
- 4. Shimizu A, Yamada K, Meehan SM, Sachs DH, Colvin RB. Acceptance reaction: intragraft events associated with tolerance to renal allografts in miniature swine. J Am Soc Nephrol. 2000;11:2371-80. (IHC-FS)
- 5. Shimizu A, Yamada K, Sachs DH, Colvin RB. Mechanisms of chronic renal allograft rejection. II. Progressive allograft glomerulopathy in miniature swine. Lab Invest. 2002;82:673-85. (IHC-FS)
- 6. Vega-López MA, Arenas-Contreras G, Bailey M, Gonzáles-Pozos S, Stokes CR, Ortega MG, et al. Development of intraepithelial cells in the porcine small intestine. Dev Immunol. 2001;8:147-58. (IHC-FS)
- 7. Jung K, Alekseev KP, Zhang X, Cheon D, Vlasova AN, Saif LJ. Altered pathogenesis of porcine respiratory coronavirus in pigs due to immunosuppressive effects of dexamethasone: implications for corticosteroid use in treatment of severe acute respiratory syndrome coronavirus. J Virol. 2007;81:13681-93. (IHC-PS)
- 8. Pescovitz MD, Lunney JK, Sachs DH. Murine anti-swine T4 and T8 monoclonal antibodies: distribution and effects on proliferative and cytotoxic T cells. J Immunol. 1985;134:37-44. (Block)
- 9. Saíz JC, Rodríguez A, González M, Alonso F, Sobrino F. Heterotypic lymphoproliferative response in pigs vaccinated with foot-and-mouth disease virus. Involvement of isolated capsid proteins. J Gen Virol. 1992;73:2601-7. (Block)
- 10. Suzuki T, Sundt TM 3rd, Mixon A, Sachs DH. In vivo treatment with antiporcine T cell antibodies. Transplantation. 1990;50:76-81. (Depletion)
- 11. Davis ME, Maxwell CV, Erf GF, Brown DC, Wistuba TJ. Dietary supplementation with phosphorylated mannans improves growth response and modulates immune function of weanling pigs. J Anim Sci. 2004;82:1882-91. (FC)
- 12. Nofrarías M, Manzanilla EG, Pujols J, Gibert X, Majó N, Segalés J, et al. Effects of spray-dried porcine plasma and plant extracts on intestinal morphology and on leukocyte cell subsets of weaned pigs. J Anim Sci. 2006;84:2735-42. (FC)
- 13. Toka FN, Nfon C, Dawson H, Golde WT. Natural killer cell dysfunction during acute infection with foot-and-mouth disease virus. Clin Vaccine Immunol. 2009;16:1738-49. (FC)
- 14. Toka FN, Nfon CK, Dawson H, Estes DM, Golde WT. Activation of porcine natural killer cells and lysis of foot-and-mouth disease virus infected cells. J Interferon Cytokine Res. 2009;29:179-92. (FC)
- 15. Martelli P, Gozio S, Ferrari L, Rosina S, De Angelis E, Quintavalla C, et al. Efficacy of a modified live porcine reproductive and respiratory syndrome virus (PRRSV) vaccine in pigs naturally exposed to a heterologous European (Italian cluster) field strain: Clinical protection and cell-mediated immunity. Vaccine. 2009;27:3788-99. (FC)
- 16. Diaz-San Segundo F, Moraes MP, de Los Santos T, Dias CC, Grubman MJ. Interferon-induced protection against foot-and-mouth disease virus infection correlates with enhanced tissue-specific innate immune cell infiltration and interferon-stimulated gene expression. J Virol. 2010;84:2063-77. (IHC-FS)
- 17. Ferrari L, De Angelis E, Martelli P, Borghetti P. Swine virus-specific cytotoxic cells producing IFNγ under different conditions of virus antigenic stimulation. Vet Res Commun. 2010;34:S63-7. (FC)
- 18. Ferrari L, Borghetti P, Gozio S, De Angelis E, Ballotta L, Smeets J, et al. Evaluation of the immune response induced by intradermal vaccination by using a needle-less system in comparison with the intramuscular route in conventional pigs. Res Vet Sci. 2011;90:64-71. (FC)
- 19. Wen K, Li G, Zhang W, Azevedo MS, Saif LJ, Liu F, et al. Development of γδ T cell subset responses in gnotobiotic pigs infected with human rotaviruses and colonized with probiotic lactobacilli. Vet Immunol Immunopathol. 2011;141:267-75. (FC)
- 20. Hester SN, Comstock SS, Thorum SC, Monaco MH, Pence BD, Woods JA, et al. Intestinal and systemic immune development and response to vaccination are unaffected by dietary (1,3/1,6)-β-D-glucan supplementation in neonatal piglets. Clin Vaccine Immunol. 2012;19:1499-508. (FC)
- 21. Oh Y, Seo HW, Han K, Park C, Chae C. Protective effect of the maternally derived porcine circovirus type 2 (PCV2)-specific cellular immune response in piglets by dam vaccination against PCV2 challenge. J Gen Virol. 2012;93:1556-62. (FC)
- 22. Wen K, Bui T, Li G, Liu F, Li Y, Kocher J, et al. Characterization of immune modulating functions of γδ T cell subsets in a gnotobiotic pig model of human rotavirus infection. Comp Immunol Microbiol Infect Dis. 2012;35:289-301. (FC)
- 23. Liu KY, Comstock SS, Shunk JM, Monaco MH, Donovan SM. Natural killer cell populations and cytotoxic activity in pigs fed mother's milk, formula, or formula supplemented with bovine lactoferrin. Pediatr Res. 2013;74:402-7. (FC)
- 24. Thorum SC, Hester SN, Comstock SS, Monaco MH, Pence BD, Woods JA, et al. Dietary (1,3/1,6)-β-D-glucan decreases transforming growth factor β expression in the lung of the neonatal piglet. Nutr Res. 2013;33:322-31 (FC)
- 25. Liu Y, Che TM, Song M, Lee JJ, Almeida JA, Bravo D, et al. Dietary plant extracts improve immune responses and growth efficiency of pigs experimentally infected with porcine reproductive and respiratory syndrome virus. J Anim Sci. 2013;91:5668-79. (FC)
- 26. Ferrari L, Borghetti P, De Angelis E, Martelli P. Memory T cell proliferative responses and IFN-γ productivity sustain long-lasting efficacy of a Cap-based PCV2 vaccine upon PCV2 natural infection and associated disease. Vet Res. 2014;45:44. (FC)
- 27. Liu P, Pieper R, Tedin L, Martin L, Meyer W, Rieger J, et al. Effect of dietary zinc oxide on jejunal morphological and immunological characteristics in weaned piglets. J Anim S. 2014;92:5009-18. (FC)
- 28. Zhu Y, Li X, Zhang W, Zhou D, Liu H, Wang J. Dose-dependent effects of Lactobacillus rhamnosus on serum interleukin-17 production and intestinal T-cell responses in pigs challenged with Escherichia coli. Appl Environ Microbiol. 2014;80:1787-98. (FC)
See More