Share a link
Introduction to ELISA
Enzyme-linked immunosorbent assay (ELISA) is a plate-based technique for detecting and quantifying an analyte of interest within a heterogeneous mixture. The advantages of ELISA are that it is quick and easy to perform, adaptable to the detection of almost any target, and can be used for high-throughput applications. ELISA is also incredibly sensitive, provided it is carefully optimized.

How does an ELISA work?
All ELISAs follow the same basic principle- the target analyte is captured on the surface of the microplate wells, the wells are blocked to prevent any non-specific binding, then the bound analyte is detected via an enzyme-based reaction. However, ELISA can be configured in several different ways, depending largely on the aims of the experiment.
What are the main ELISA formats?
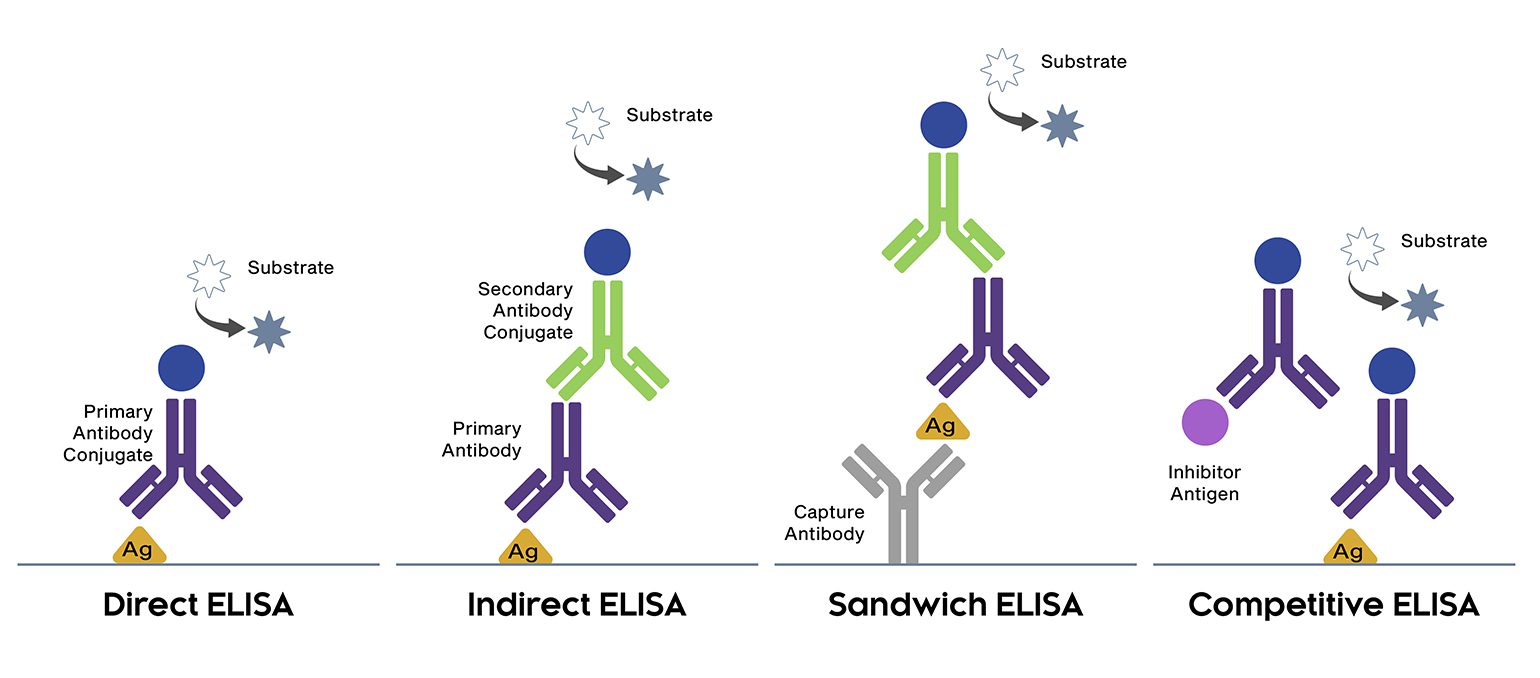
ELISA is grouped into four main categories according to how it is configured. During a direct ELISA, the analyte is bound directly to the microplate wells and detected using an analyte-specific (primary) antibody labeled with an enzyme such as horseradish peroxidase (HRP) or alkaline phosphatase (AP). The main advantage of direct ELISA is its short workflow. However, direct ELISA has only limited sensitivity since it does not include any form of signal amplification.
Like direct ELISA, indirect ELISA also involves binding the analyte straight onto the microplate wells. Yet, following the addition of the primary antibody, an enzyme-labeled secondary antibody is used for detection. Indirect ELISA is more sensitive than direct ELISA because multiple secondary antibodies can bind each primary antibody to provide signal amplification. It can also be more flexible, since labeled primary antibodies are not always available. A disadvantage of indirect ELISA is that it introduces the potential for cross-reactivity between secondary antibodies and the target analyte.
Sandwich ELISA is the most widely used ELISA format due to its superior specificity, which is achieved by using matched antibody pairs for analyte capture and detection. After the first analyte-specific antibody has bound to the microplate wells, the sample is added, then a labeled analyte-specific antibody is used for detection. Alternatively, sandwich ELISA can use a labeled secondary antibody for indirect detection; here, cross-adsorbed secondary antibodies are essential to prevent non-specific secondary antibody binding to the capture antibody.
We offer a broad range of secondary antibodies that have been cross-adsorbed against purified immunoglobulins and serum proteins from multiple species. Popular products for ELISA include donkey secondaries raised against mouse, goat, or rabbit IgG, and goat secondaries for detecting mouse, human, rabbit, or chicken antibodies.
Direct, indirect, and sandwich ELISA can all be converted to a competitive ELISA. In this format, the target analyte - which may be either a protein or an antibody - competes with a reference for binding to a known amount of labeled antibody or protein, respectively. Usually, samples containing a high concentration of the target analyte yield a lower signal than those where the target analyte is more abundant.
How are biotinylated secondary antibodies used for signal amplification?
Although enzyme-labeled secondary antibodies can enhance the ELISA signal, detecting scarce analytes sometimes requires an additional protocol step known as labeled streptavidin-biotin (LSAB) signal amplification. In this situation, the enzyme-labeled secondary antibodies are replaced by biotinylated equivalents then a labeled streptavidin complex is used for detection. This approach increases the enzyme:antibody ratio for additional signal amplification.
Our labeled streptavidin products include streptavidin-HRP and streptavidin-AP and are easily incorporated into the ELISA workflow.
What type of readout does ELISA produce?
ELISA commonly employs colorimetric detection, whereby the enzyme-catalyzed conversion of a substrate into a soluble colored product is measured using a spectrophotometer. 3,3′,5,5′-tetramethylbenzidine (TMB) is widely used as a substrate for HRP, producing a yellow readout measurable at 450 nm after the reaction has been stopped with an acid (e.g., sulfuric acid or hydrochloric acid). Alkaline phosphatase instead uses p-nitrophenyl phosphate (pNPP), which yields a signal between 405 nm and 410 nm.
Chemiluminescent detection is a popular alternative to measuring absorbance that typically relies on HRP-labeled antibodies for catalyzing the production of measurable light using a luminol-based substrate. While chemiluminescent detection can afford greater sensitivity than colorimetric detection, it requires specialized reagents and the use of a dedicated reader.
A variation on ELISA known as fluorescence-linked immunosorbent assay (FLISA) uses fluorophore-labeled antibodies in place of enzyme reporters for detection. An advantage of this approach is that it requires less manual intervention than colorimetric or chemiluminescent ELISA, making it popular for high-throughput applications. We include FLISA within our QC testing strategy, where it provides additional confidence in antibody performance.
How does ELISA enable analyte quantification?
Unlike many other immunoassay techniques, ELISA allows researchers to quantify the analyte of interest. This is accomplished by serially diluting a known amount of the target analyte to generate a standard curve against which the concentration of any test samples can be calculated. For accurate quantification of ELISA data, the standard should be diluted in the same matrix as the sample material and the standard curve run on every plate, ideally in duplicate or triplicate. It is equally important that a consistent batch of material is used throughout each assay to avoid intraplate variability.
Our mouse and human standards include many different antibody isotypes and subtypes, as well as specific antibody light chains.