Share a link
Whole Molecule Versus Fragmented Antibodies
Antibodies are essential tools for a broad range of research applications, including established techniques such as enzyme-linked immunosorbent assay (ELISA), Western blot, and immunohistochemistry (IHC). Historically, whole antibody molecules have been the only available option for performing these methods. However, fragmented antibodies are increasingly popular and can offer several advantages over their larger counterparts for certain uses.
What is the basic structure of an antibody?
Antibodies are complex proteins produced by B cells during the adaptive immune response. They comprise five main isotypes (IgG, IgA, IgD, IgE and IgM) according to the heavy chain they contain, with IgG being the most abundant. A typical IgG antibody consists of two identical heavy chains and two identical light chains, held together by disulfide bonds. Each antibody chain is folded into constant and variable domains, with heavy chains containing three constant domains and one variable domain while light chains contain just one of each domain type. The antibody fragment crystallizable (Fc) region, consisting of two constant domains of each antibody heavy chain, functions to promote antigen removal by interacting with components of the immune system (e.g., Fc receptors on immune cells). It is attached by a flexible hinge region to two antigen binding fragments (Fabs), each consisting of two constant and two variable domains, with the latter having a pivotal role in antigen recognition.
Why are antibodies important for research?
Antibodies are used for research due to their unique ability to specifically detect any analyte within a heterogeneous mixture. This specificity results from mechanisms known as V-D-J recombination and junctional diversification, which occur in early B cell development, as well as somatic hypermutation and affinity maturation, which take place upon B cell activation and during progression of the adaptive immune response. By using antibodies for monitoring analytes of interest in sample material, researchers can compare target expression in conditions of health and disease to develop effective treatments for conditions including cancer, Alzheimer’s disease, and various autoimmune disorders.
How are whole antibody molecules used in a research setting?
A typical immunoassay workflow involves performing a sequential series of steps, beginning with blocking to prevent unwanted background signal. Next, samples are incubated with primary antibodies that recognize and bind any target analytes, prior to washing and incubation with labeled secondary antibodies that provide a measurable readout. Depending on the target of interest and the chosen immunoassay technique, it may be necessary to perform additional steps. These include Fc blocking during IHC to prevent antibody binding to Fc receptors on immune cells, and permeabilization during immunocytochemistry to enable antibodies to access cytoplasmic or nuclear antigens.
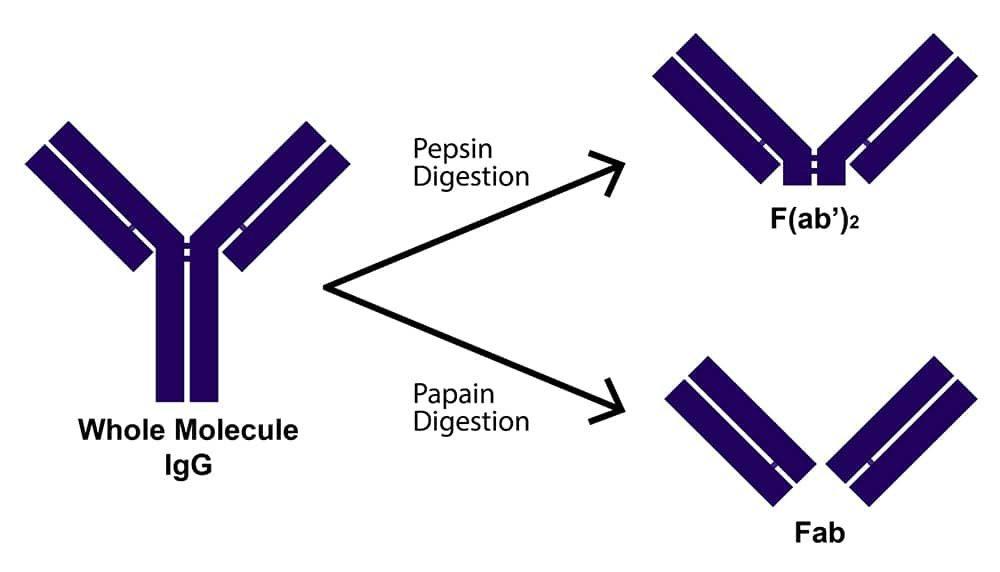
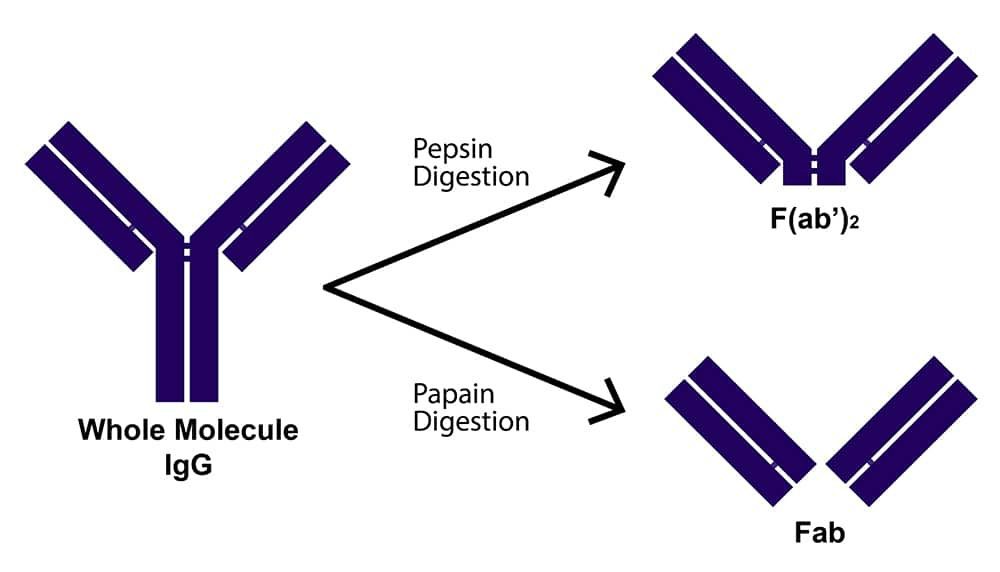
What are fragmented antibodies?
Antibody fragments are formed by reducing the hinge region disulfide bonds of whole antibody molecules and/or performing proteolytic digestion. Common fragment types include F(ab')2, which is generated by pepsin digestion and comprises two antigen binding regions joined at the hinge by disulfide bonds; Fab', which is formed by reducing F(ab')2 and, like F(ab')2, contains a small portion of the Fc region; and Fab, which is produced by papain digestion and does not encompass any of the Fc region. These have molecular weights of approximately 110, 55, and 50 kDa, respectively, while a whole antibody molecule has a molecular weight of around 150 kDa.
What are the advantages of using fragmented antibodies?
Fragmented antibodies offer several advantages over whole antibody molecules for use in certain applications. For example, fragments lacking an Fc region are unable to bind Fc receptors, thereby eliminating the need for Fc receptor blocking during IHC and avoiding Fc-mediated events such as antibody-dependent cell-mediated cytotoxicity (ADCC) during functional assays. The smaller size of fragmented antibodies also provides more efficient penetration of cells and tissue sections for better visualization of subcellular structures. Additionally, for solid phase applications, antibody fragments promise higher sensitivity through reduced steric hindrance.
SouthernBiotech offers a wide range of F(ab’)2 and Fab fragmented secondary antibodies specific to mouse, human, rabbit, rat, goat, sheep, and hamster immunoglobulins.